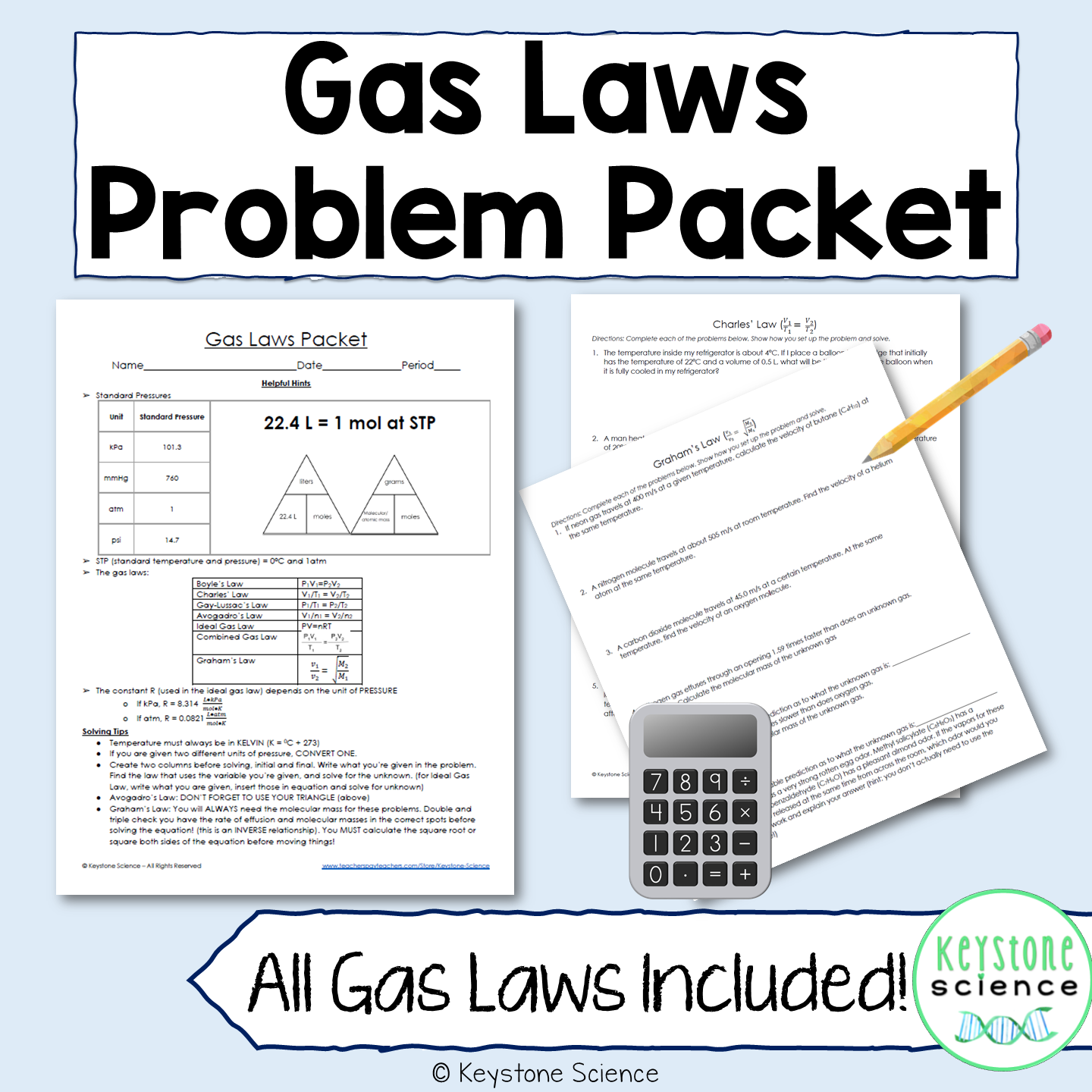
Mixed Gas Law Problems Answers . What is the partial pressure of. Use the ideal gas law pv = nrt, to. Boyle’s law, charles’s law, and avogadro’s law, as well as the combined gas law. the following practice problems are to master to topics on the ideal gas laws: A gas occupies 3.5l at 2.5 mm hg pressure. What is the volume at 10 mm hg at the same temperature? solve each of the following problems, being sure to show your work and include all proper units. mixed gas laws worksheet 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292. mixed extra gas law practice problems (ideal gas, dalton’s law of partial pressures, graham’s law) 1. A mixture of 40.0 g of oxygen and 40.0 g of helium has a total pressure of 0.900 atm.
from classful.com
What is the partial pressure of. A gas occupies 3.5l at 2.5 mm hg pressure. Boyle’s law, charles’s law, and avogadro’s law, as well as the combined gas law. the following practice problems are to master to topics on the ideal gas laws: mixed gas laws worksheet 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292. solve each of the following problems, being sure to show your work and include all proper units. mixed extra gas law practice problems (ideal gas, dalton’s law of partial pressures, graham’s law) 1. A mixture of 40.0 g of oxygen and 40.0 g of helium has a total pressure of 0.900 atm. What is the volume at 10 mm hg at the same temperature? Use the ideal gas law pv = nrt, to.
Chemistry Gas Laws Packet Massive Problem Set ANSWER KEY INCLUDED Classful
Mixed Gas Law Problems Answers mixed extra gas law practice problems (ideal gas, dalton’s law of partial pressures, graham’s law) 1. solve each of the following problems, being sure to show your work and include all proper units. the following practice problems are to master to topics on the ideal gas laws: Boyle’s law, charles’s law, and avogadro’s law, as well as the combined gas law. mixed extra gas law practice problems (ideal gas, dalton’s law of partial pressures, graham’s law) 1. A mixture of 40.0 g of oxygen and 40.0 g of helium has a total pressure of 0.900 atm. Use the ideal gas law pv = nrt, to. What is the partial pressure of. mixed gas laws worksheet 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292. A gas occupies 3.5l at 2.5 mm hg pressure. What is the volume at 10 mm hg at the same temperature?
From www.slideshare.net
Mixed Gas Law Problems Mixed Gas Law Problems Answers What is the partial pressure of. solve each of the following problems, being sure to show your work and include all proper units. Use the ideal gas law pv = nrt, to. mixed extra gas law practice problems (ideal gas, dalton’s law of partial pressures, graham’s law) 1. What is the volume at 10 mm hg at the. Mixed Gas Law Problems Answers.
From worksheets.decoomo.com
30++ Mixed Gas Laws Worksheet Answers Worksheets Decoomo Mixed Gas Law Problems Answers A gas occupies 3.5l at 2.5 mm hg pressure. A mixture of 40.0 g of oxygen and 40.0 g of helium has a total pressure of 0.900 atm. Boyle’s law, charles’s law, and avogadro’s law, as well as the combined gas law. What is the volume at 10 mm hg at the same temperature? What is the partial pressure of.. Mixed Gas Law Problems Answers.
From dxohjhnpb.blob.core.windows.net
Mixed Gas Laws Problems Answers at Stephanie Neal blog Mixed Gas Law Problems Answers A mixture of 40.0 g of oxygen and 40.0 g of helium has a total pressure of 0.900 atm. the following practice problems are to master to topics on the ideal gas laws: Boyle’s law, charles’s law, and avogadro’s law, as well as the combined gas law. mixed gas laws worksheet 1) how many moles of gas occupy. Mixed Gas Law Problems Answers.
From www.scribd.com
Gas Laws Packet 2 ANSWERS Gases Statistical Mechanics Mixed Gas Law Problems Answers Use the ideal gas law pv = nrt, to. A gas occupies 3.5l at 2.5 mm hg pressure. A mixture of 40.0 g of oxygen and 40.0 g of helium has a total pressure of 0.900 atm. What is the partial pressure of. solve each of the following problems, being sure to show your work and include all proper. Mixed Gas Law Problems Answers.
From www.studocu.com
Gas Laws Worksheet answer key Gas Laws Worksheet atm = 760 mm Hg = 101 kPa= 760 .0 torr Boyle Mixed Gas Law Problems Answers A gas occupies 3.5l at 2.5 mm hg pressure. mixed gas laws worksheet 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292. What is the volume at 10 mm hg at the same temperature? the following practice problems are to master to topics on the ideal gas. Mixed Gas Law Problems Answers.
From studydblang99.z21.web.core.windows.net
Mixed Gas Laws Worksheet Answers Mixed Gas Law Problems Answers mixed extra gas law practice problems (ideal gas, dalton’s law of partial pressures, graham’s law) 1. What is the partial pressure of. What is the volume at 10 mm hg at the same temperature? mixed gas laws worksheet 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292.. Mixed Gas Law Problems Answers.
From classful.com
Chemistry Gas Laws Packet Massive Problem Set ANSWER KEY INCLUDED Classful Mixed Gas Law Problems Answers mixed extra gas law practice problems (ideal gas, dalton’s law of partial pressures, graham’s law) 1. Boyle’s law, charles’s law, and avogadro’s law, as well as the combined gas law. mixed gas laws worksheet 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292. the following practice. Mixed Gas Law Problems Answers.
From worksheets.decoomo.com
30++ Mixed Gas Laws Worksheet Answers Worksheets Decoomo Mixed Gas Law Problems Answers What is the partial pressure of. solve each of the following problems, being sure to show your work and include all proper units. Use the ideal gas law pv = nrt, to. A gas occupies 3.5l at 2.5 mm hg pressure. What is the volume at 10 mm hg at the same temperature? mixed gas laws worksheet 1). Mixed Gas Law Problems Answers.
From studylib.net
Mixed Gas Law Worksheet Answers available at end Name (show Mixed Gas Law Problems Answers mixed extra gas law practice problems (ideal gas, dalton’s law of partial pressures, graham’s law) 1. What is the partial pressure of. mixed gas laws worksheet 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292. A gas occupies 3.5l at 2.5 mm hg pressure. What is the. Mixed Gas Law Problems Answers.
From materialliboster.z19.web.core.windows.net
Mixed Gas Laws Worksheet Answers Mixed Gas Law Problems Answers A gas occupies 3.5l at 2.5 mm hg pressure. the following practice problems are to master to topics on the ideal gas laws: A mixture of 40.0 g of oxygen and 40.0 g of helium has a total pressure of 0.900 atm. Use the ideal gas law pv = nrt, to. mixed extra gas law practice problems (ideal. Mixed Gas Law Problems Answers.
From lessonfullkortig.z1.web.core.windows.net
Mixed Gas Law Worksheets Mixed Gas Law Problems Answers mixed gas laws worksheet 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292. mixed extra gas law practice problems (ideal gas, dalton’s law of partial pressures, graham’s law) 1. solve each of the following problems, being sure to show your work and include all proper units.. Mixed Gas Law Problems Answers.
From printablewandyl9o.z22.web.core.windows.net
Gas Law Practice Problems With Answers Pdf Mixed Gas Law Problems Answers What is the partial pressure of. mixed extra gas law practice problems (ideal gas, dalton’s law of partial pressures, graham’s law) 1. A gas occupies 3.5l at 2.5 mm hg pressure. solve each of the following problems, being sure to show your work and include all proper units. Use the ideal gas law pv = nrt, to. A. Mixed Gas Law Problems Answers.
From studylib.net
MIXED GAS LAWS WORKSHEET Mixed Gas Law Problems Answers Use the ideal gas law pv = nrt, to. mixed extra gas law practice problems (ideal gas, dalton’s law of partial pressures, graham’s law) 1. Boyle’s law, charles’s law, and avogadro’s law, as well as the combined gas law. What is the volume at 10 mm hg at the same temperature? solve each of the following problems, being. Mixed Gas Law Problems Answers.
From dxohjhnpb.blob.core.windows.net
Mixed Gas Laws Problems Answers at Stephanie Neal blog Mixed Gas Law Problems Answers mixed gas laws worksheet 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292. the following practice problems are to master to topics on the ideal gas laws: A gas occupies 3.5l at 2.5 mm hg pressure. solve each of the following problems, being sure to show. Mixed Gas Law Problems Answers.
From studylibfreytag.z21.web.core.windows.net
Combined Gas Law Practice Worksheet Answers Mixed Gas Law Problems Answers the following practice problems are to master to topics on the ideal gas laws: What is the partial pressure of. mixed extra gas law practice problems (ideal gas, dalton’s law of partial pressures, graham’s law) 1. solve each of the following problems, being sure to show your work and include all proper units. What is the volume. Mixed Gas Law Problems Answers.
From www.studocu.com
Mixed Gas Laws Worksheet Grey Mixed Gas Laws Worksheet How many moles of gas occupy 98 L at a Mixed Gas Law Problems Answers A mixture of 40.0 g of oxygen and 40.0 g of helium has a total pressure of 0.900 atm. What is the partial pressure of. the following practice problems are to master to topics on the ideal gas laws: solve each of the following problems, being sure to show your work and include all proper units. mixed. Mixed Gas Law Problems Answers.
From www.housview.com
Mixed Gas Laws Worksheets Answers Mixed Gas Law Problems Answers What is the volume at 10 mm hg at the same temperature? solve each of the following problems, being sure to show your work and include all proper units. Use the ideal gas law pv = nrt, to. Boyle’s law, charles’s law, and avogadro’s law, as well as the combined gas law. mixed extra gas law practice problems. Mixed Gas Law Problems Answers.
From studydblang99.z21.web.core.windows.net
Mixed Gas Laws Worksheet Answers Mixed Gas Law Problems Answers mixed gas laws worksheet 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292. mixed extra gas law practice problems (ideal gas, dalton’s law of partial pressures, graham’s law) 1. A mixture of 40.0 g of oxygen and 40.0 g of helium has a total pressure of 0.900. Mixed Gas Law Problems Answers.